How Do You Choose Partners to Develop the Product?
Marketing Approval process
Marketing Approval Procedures
In Europe, there are different procedures which depending on the type of pharmaceutical product and type of disease where you can apply for approval in multiple EU countries at once, [ EC diagram, EUPATI Procedures ] Once approved you may need to apply for changes to Approved Marketing Authorizations according to their complexity such as Type 1, Type 2 in the European Union.
For marketing approval, you will need to complete and submit a series of files as a Common Technical Dossier (CTD)
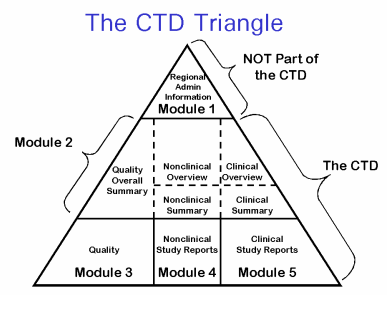
The information & format how you submit the information (CTD, paper/electronic, etc) depends on the country.
Electronic example sections
- open source eCTD demo application template
- eCTD indexer.
Regulatory Advice
TOPRA (European regulatory membership organisation) has tips for preparing documentation. In most countries, you need to plan for reimbursement too (e.g. NICE in UK, G-BA in Germany). Very innovative medicines can be accelerated (PRIME in EU, Breakthrough in US, etc). Some approvals outside the European Union have used the Article 58 joint opinion to bring medicines to market.
In the UK, NICE offer scientific advice for a fee to help people with no previous experience develop a product. Between 2009 - 2015 they advised on 166 projects which involved medicinal products (against an approximate 7000 trials across the EU), with the majority of advice being sought at phase 2 and phase 3.
National regulators may also provide free advice on websites or in person. [ EUPATI Marketing application]
The figures below are a cause of disagreement between healthcare or scientific professionals and the pharmaceutical industry but these are what companies are claiming it costs. As larger and more open compound discovery and manufacturing processes are computerised / automated this will continue to reduce costs.
