Step 2: Establish the existence of an Outbreak
How are Outbreaks Recognized?
Outbreaks may come to the attention of public health via various sources, including direct reports from clinicians, the public or the media. As well, epidemiologists continually analyze routine surveillance data for unusual patterns of disease.
Physicians
Most outbreaks come to the attention of health authorities because an alert clinician is concerned enough to call the health department (CDC, 2012, p. 413)
Several detected diseases are new and may not fall under mandatory reporting requirements unless the regulations demand that any outbreak or situation that threatens public health be reported to public health authorities. Some of these diseases cannot be confirmed in a laboratory as the tests either do not yet exist or are not available in current practice. For example, amnesic shellfish poisoning (due to domoic acid, for which there is no laboratory test for humans) was discovered in 1987, via physician reports. As a second example, the first report of what is now known as AIDS came from physicians who had seen an unusual pattern of disease in men in California. This outbreak was reported in MMWR in 1982.
Affected Persons
Cases themselves, or members of a group that is affected, are frequently the sources for reporting an outbreak. For example, the organizer of a banquet can alert a public health department of the occurrence of enteric diseases among the guests.
Media
Media impact on the disease outbreak is a dynamic process. It has its greatest effect in reducing disease transmission at the initial stage of an outbreak
Information coming from those media can lead ill individuals to consult doctors and lead physicians to search for the etiologic agent and report cases to public health authorities. (Xiao, Y et al, 2015)
Surveillance Data
Frequent and detailed analysis of routine surveillance data can be important in detecting smaller or geographically dispersed “hidden outbreaks” that may otherwise escape notice.
These changes can be identified by the routine analysis of data using the characteristics of time (e.g., in weeks, months or four-week periods, etc.), place (e.g., municipalities, city areas, etc.), and persons (e.g., sex, age groups, sub-types of pathogenic agents [Salmonella serotypes…], etc.). These data can be presented as tables (which contain detailed numerical values) or illustrated using figures (graphics or diagrams). (Oxford, 2014)
Surveillance
Surveillance data identify the baseline occurrence of disease within the population and such data, therefore, are used to monitor reports for changes. In Canada, public health surveillance systems for many notifiable diseases are based on laboratory diagnosis.
Surveillance
The systematic ongoing collection, collation, and analysis of data and the timely dissemination of information to those who need to know so that action can be taken.
Outbreaks may be detected when health department staff conduct regular, timely analysis of surveillance data that reveals an increase in reported cases or an unusual clustering of cases by time and place (CDC, 2012, p. 413)
The frequency of analysis and the time interval chosen for aggregation of data will depend on the disease. Unfortunately, for each disease there is the risk of selecting time intervals that are too short and raising false alarms; or selecting time intervals that are too long, and not detecting an outbreak until after the fact. Surveillance drives the cycle of public health prevention as illustrated in the figure below.
Cycle of Public Health Prevention
Even the simplest surveillance program will be able to tabulate data by age group, sex, province and reporting period. A breakdown of data by these and other factors is necessary to find meaningful patterns. The more data available, especially for the agents that cause infectious diseases, the easier it is to find patterns; otherwise, an outbreak will be "drowned out" by the endemic level of disease. For example, routine surveillance of salmonellosis shows that this is a very common infection. However, when tabulating the number of cases by Salmonella serotype, week of reporting and province, unusual serotypes stand out.
In infectious diseases, there is also the "iceberg" phenomenon -infectious diseases are like icebergs, in that what is visible above water (reported cases) represents only a fraction of the total ice mass (total infections) hidden from view.
Reasons for Increased Reporting of Cases
Even if the actual number of cases that are reported exceeds the number expected, this increase does not necessarily indicate the presence of an outbreak. The increase could be an artefact (how the data were collected; new lab tests, etc.). A good understanding of surveillance programs is needed to be able to interpret departures from the norm.
Potential reasons for change in case reporting
- Media attention to information released by public health authorities can lead affected individuals to consult a physician, whereas they would not have done so under normal circumstances
- Changes in the level of clinical practice can lead doctors to request additional tests
- A new laboratory test (or a more sensitive one) may be introduced
- Changes within the department of public health
- active search for cases
- modification of the case definition
Other factors may actually lead to a decrease in the number of reported cases. A delay in the delivery of surveillance data from a region can cause a slump in the number of reports, although this is frequently followed by an increase when the data finally arrive. Some laboratories accumulate the reports to be transmitted to the department of public health and send them in batches, causing fluctuations in the numbers. Surveillance data are traditionally compiled and analyzed according to the date of receipt of reports, which is subject to this type of variation. Having the dates that specimens were taken from the cases or the dates of onset of symptoms makes it easier to identify real increases. A cluster of sample collection dates in a number of patients, or of the onset of disease dates, would be hard to explain by an artefact and suggest the possible presence of an outbreak.
Pseudo-outbreaks
Pseudo-outbreaks are defined as an increase in identified organisms but without evidence of infection. They may represent a false cluster of real infections or a real cluster of false infections. (Sood G. Perl T., 2016)
The former is similar to the surveillance artifacts described above (e.g., the arrival in a hospital of a new doctor who is interested in a particular infection and who starts to search for it more intensively).
Because pseudo-outbreaks generally represent contamination, identification of the source is important to prevent inappropriate treatment and additional testing in patients who do not have a true infection. Between 1965 and 2010, 72 clusters of pseudobacteremia have been published, 22 cases of pseudomeningitis, and 49 cases of pseudopneumonia. Pseudo-infections most commonly present as pseudobacteremias. Pseudobacteremias occur in the setting of contaminated culture media, contaminated antiseptics, contaminated blood culture vials, or inadequate disinfection of the analyzer. Although less common, pseudomeningitis has significant sequelae and has been due to contamination of procedure kits or culture media. Pseudopneumonia was most often due to mycobacterial species and was most often related to bronchoscopy. This type of occurrence should be considered as serious as a real outbreak because of the problems to which it could lead: unnecessary treatment with antibiotics, the wrong antibiotic, inappropriate additional investigations, etc. Investigating them helps to uncover inadequate procedures, defective equipment, or contaminated products and from there, to make the required corrections (Sood G. Perl T., 2016)
A pseudo-outbreak can also occur due to an error in clinical diagnosis. The simultaneous occurrence of two diseases with a similar clinical presentation, but of different etiology, can also be confused with an outbreak (e.g., influenza and other respiratory viruses), which underlines the desirability of confirming at least the initial cases by laboratory analyses.
An apparent increase in cases in a healthcare institution, which could lead one to believe in an outbreak of nosocomial infections (e.g., Legionella, methicillin-resistant Staphylococcus aureus [MRSA] infections, etc.), could be due, instead, to an increased incidence in the community, which demonstrates the importance of case definitions that take into account the length of stay in the institution.
Recognizing surveillance artifacts and pseudo-outbreaks early on can avoid unnecessary investigations.
Observed Versus Expected Frequency
Determining the background frequency (incidence rate) is relatively simple when the etiological agent is known, the tests confirming the agent in question are conducted on a routine basis and surveillance data are complete. For example, the incidence of measles in Canada is low and almost all cases will be recognized and confirmed through enhanced surveillance. However, this is not the case for many other infectious diseases. What is considered to be an "excess" is rather subjective and imprecise, and that decision is often made by "eyeballing" the data or using common sense. If that excess is very high and sudden, this usually suggests an outbreak. Where there is marginal excess, it is necessary to analyze more data on the known cases or to wait for additional surveillance data.
This comparison depends on the availability of previous data for the population in question. It is preferable to compare current numbers with those of previous years during the same periods, to take possible seasonal variations into account. Usually, such a comparison is limited to the previous five years. The incidence of a disease can change steadily over a number of years (what is called a secular trend). It is hard to compare current numbers with those, for instance, of ten years before, as several elements related to the surveillance of that disease (e.g., case definition, completeness of reports, mode of data compilation, etc.) could have changed with time. Also consider that some diseases have annual or multi-year outbreak cycles (e.g., pertussis, influenza, etc.).
Where there is no previous data for the location of interest, the data for neighboring regions, the province, or the country could be used for comparison. It may be possible to conduct a quick survey of several physicians, clinics, or hospitals to determine if they had observed more cases than usual.
The laboratory confirmation of reported cases frequently takes days or weeks. If the interim data indicates an obvious increase of confirmed cases, in the absence of a significant decrease in reporting delays, there is a reason to suspect an outbreak.
It is important to calculate the incidence rates, if possible, for a comparison of the occurrence of disease across time, as a population (denominator) can change. Using only the counts of disease (numerators) could lead to misinterpretation, although this is not likely to happen over the short term. The choice of an appropriate denominator is also crucial in the surveillance of nosocomial infections (e.g., the number of patient-days of stay in hospital is usually preferable to the numbers of hospital admissions, as it is a better reflection of risk).
When comparing the rates of different regions, verify if the age distribution is different. This is particularly important if the disease in question has a predilection for certain age groups. If so, standardize the rates to make this comparison more equitable. The same logic applies to other personal characteristics.
Some statistical techniques can be applied to surveillance data to evaluate changes in frequency or incidence. By considering these data as samples through time, the apparent increases of health-related events can be evaluated for their statistical significance. Applying a confidence interval (CI) - of 95%, for example - to these samples can allow a determination of whether the observed differences can be due only to chance. The term "aberration" is used to describe a significant deviation from usual frequency. Such a deviation does not necessarily signify that there is an outbreak. A graphical representation of the data is very useful, especially when there is comparable data from previous years. The graph below provides an example.
Influenza-like Illness Surveillance Network (ILINet) (2009 - 2020)
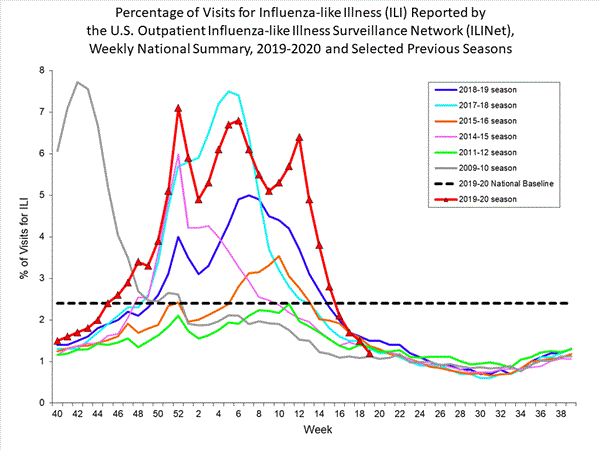
ILI = influenza like illness
References
Centers for Disease Control and Prevention (CDC), National Center fo Immunization and Respiratory diseases (NCIRD). (2020, June 26). Weekly U.S. Influenza Surveillance Report. https://www.cdc.gov/flu/weekly/#ILINet
Sood, G. & Perl, T. (2016). Outbreaks in Health Care Settings. Infect Dis Clin N Am, 30, 661–687. http://dx.doi.org/10.1016/j.idc.2016.04.003.
U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC), Office of Workforce and Career Development. (2012). Principles of epidemiology in public health practice: an introduction to applied epidemiology and biostatistics (3rd ed.).
Weiss, N. & Koepsell, T. (2014). Epidemiologic Methods: Studying the Occurrence of Illness (2nd ed.). Oxford University Press
World Health Organization (WHO). Tobacco Free Initiative (TFI). https://www.who.int/tobacco/surveillance/about_surveillance/en/
Xiao, Y., Tang, S. & Wu, J. (2015). Media impact switching surface during an infectious disease outbreak. Sci Rep 5, 7838. https://doi.org/10.1038/srep07838
